Coagulation and flocculation are two important parts of wastewater and drinking water treatment.
In this guide, we’ve shared everything you need to know about these treatment processes, including why they’re used, how they work, and what results they produce.
📌 Key Takeaways:
- Coagulation and flocculation are two types of processes used in wastewater drinking water treatment plants.
- The purpose of these processes is to improve water quality by reducing turbidity and removing small suspended solids. These particles are easiest to remove when they’re clumped together in groups.
- There are numerous types of organic and inorganic coagulants that can be used in water treatment, each with their own pros and cons.
Table of Contents
- 🔎 Coagulation And Flocculation: Quick Overview
- 🤔 What Is The Purpose of Coagulation and Flocculation?
- 📖 Coagulation And Flocculation: How They Work
- 🆚 Organic Vs Inorganic Coagulants
- 📋 Common Types of Coagulants Used in Water Treatment
- 🧐 Are Coagulation And Flocculation Always Needed?
- 📑 Final Word
- ❔ Coagulation And Flocculation: FAQ
🔎 Coagulation And Flocculation: Quick Overview
Just looking for a quick overview of coagulation and flocculation?
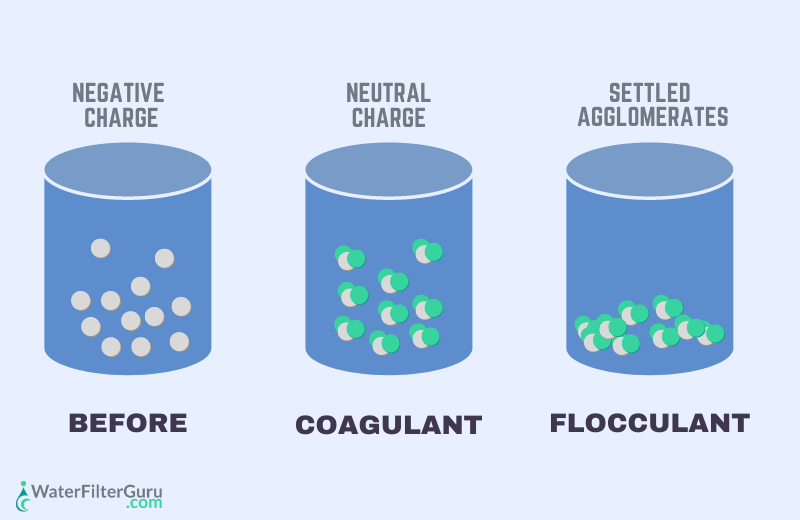
Coagulation is the process of adding chemicals to water, causing them to bind together and form ‘flocs’.
Flocculation is the process of adding flocculant to water, which further encourages floc formation and increases the floc sizes, making them easier to remove.
The clumps of particles sink to the bottom of the treatment chamber, where they can be removed from the water at a later stage.

🤔 What Is The Purpose of Coagulation and Flocculation?
Before we look in detail at the coagulation and flocculation process, you might be wondering – why is water treated with this method?
The main purpose of coagulation and flocculation is to treat turbid (hazy or cloudy) water, thus improving water quality. This is especially key for wastewater treatment, and can reduce organic loads and suspended solids by up to 90%.
Suspended solids are commonly found in the majority of surface water supplies and all wastewater supplies. These particles are negatively charged in their suspended form in the water, which causes them to repel each other and prevents them from forming clumps.
The easiest way to encourage these particles to clump together is to add coagulant chemicals. These chemicals neutralize the charge of suspended solids, and flocculation causes them to grow bigger and clump together, so that it’s easier to remove them from the liquid.
Removing visible suspended particles from water simplifies later stages of water treatment, especially disinfection. By reducing water’s turbidity, coagulation/flocculation minimizes the amount of chlorine (or similar disinfection chemicals) that must be added to the water, helping to save money and make the water safer.
📖 Coagulation And Flocculation: How They Work
Let’s take a look at the coagulation and flocculation processes in more detail.
How Does Coagulation Work?
The coagulation process involves adding chemical coagulants to water to encourage suspended particles to clump together.
This chemical process neutralizes the particles’ charges, causing them to form micro-flocs. When enough coagulant is added, there’s a defined difference between the clumped micro-flocs and the clear water surrounding them. It’s important not to add too much coagulant, which will have the opposite effect and cause the suspended particles to repel one another.
During the coagulation process, the coagulant needs to be mixed rapidly to properly disperse it throughout the entire volume of water, enhance floc growth, and promote particle collisions.
How Does Flocculation Work?
After coagulation, the flocculation process is used to increase the sizes of the suspended particles (which have now been neutralized) and further promote clumping. The agents that encourage particles to clump together, making them easy to remove from water, are flocculants.
During flocculation, the micro-flocs grow larger, forming suspended pin-flocs, which are visible to the eye. These pin-flocs produce even larger flocs, called macro-flocs, when they collide during the process.
At this stage of water treatment, polymers may be added to strengthen the floc and increase its settling weight, making it easier to remove.
Eventually, the flocs will achieve optimum strength and size, and are ready to be separated from the water. The stage that follows this process is usually sedimentation.
👨🔧 Want to learn more about the stages of the water treatment process? Check out this article.
🆚 Organic Vs Inorganic Coagulants
For use in drinking water treatment and wastewater treatment, there are two common types of coagulants: organic coagulants and inorganic coagulants.
Organic Coagulants
Organic coagulants include polytannate, polyamines, and poly DADMAC (diallyl dimethyl ammonium chloride.
Pros:
The advantages of organic coagulants include:
- They’re metal and hydroxide-free and provide essentially the same results as inorganic coagulants.
- They can remove some of the organic matter that chlorine reacts with during disinfection to form health-harmful byproducts. This should hopefully reduce the disinfection byproducts produced in the water.
- They’re corrosive and don’t affect pH.
Cons:
Some of the setbacks of using organic coagulants include:
- They cost more per unit.
- They produce low-density floc, which doesn’t always have good settling abilities.
- If the charge demand is high, high dosages must be used.

Inorganic Coagulants
Inorganic coagulants include various forms of aluminum (aluminum sulfate, sodium aluminate) and iron coagulants (ferrous and ferric sulfate, ferric chloride).
Pros
The advantages of using inorganic compounds for drinking water and wastewater treatment include:
- They can also remove a number of organic particles that react with chlorine (often used to disinfect drinking water) and produce unwanted by-products with health effects.
- They enhance the formation of micro-flocs by enabling the formation of hydrated inorganic hydroxides.
- They’re affordable and widely available, making them ideal for large-scale use.
Cons:
There are a few disadvantages to be aware of for these types of coagulants:
- The floc they produce is metal-rich, so it needs to be carefully and consciously disposed of.
- They affect water’s pH, and because the effectiveness of coagulation relies on a certain pH, pH control must be employed alongside their use.
📋 Common Types of Coagulants Used in Water Treatment
Let’s look in more detail at the most common types of coagulants that are used in drinking water and wastewater treatment.
Aluminum Sulfate
The most commonly used aluminum coagulant is aluminum sulfate. In its solid form, this coagulant is found in various options, including kibbled, ground, and block. It can also be purchased in liquid form.
Aluminum sulfate is acidic. When it’s introduced into water, the water’s natural alkalinity reacts with the coagulant’s acidity, resulting in the formation of an aluminum hydroxide floc.
Ferric Sulfate
The most widely-used iron coagulant is ferric sulfate. This coagulant, usually used alongside chlorine, has one key advantage over aluminum sulfate: it can produce a denser floc that’s easier to remove during the sedimentation stage.
One downside of using ferric sulfate, however, is that it’s more difficult to dissolve, largely because its hydroxide sludge formation is notably heavier.
Sodium Aluminate
Sodium aluminate is another type of aluminum coagulant. It’s made when aluminum oxide and sodium oxide are combined, and there are different forms available – the liquid forms contain around 30% sodium aluminate on average, while the solid forms contain around 70-80% sodium aluminate.
A benefit of using sodium aluminate instead of an iron coagulant is that it produces a much lower volume of chemical sludge. It also increases water’s pH and alkalinity, so hydroxides and lime don’t need to be added to further manage pH.
Ferric Chloride
Ferric chloride is both a coagulant and a flocculant, and is a second choice to ferric sulfate.
Using ferric chloride has a few advantages – it’s better for cold water since it encourages faster sedimentation. But chloride makes water more corrosive, so despite its versatility, this coagulant is the least popular choice in most scenarios.
There are other coagulants available if none of the above are suitable for specific applications. For example, biopolymer coagulants may be favored by water treatment plants that wish to employ safer, more environmentally-friendly practices because they’re less toxic and produce less sludge.
🧐 Are Coagulation And Flocculation Always Needed?
So, are coagulation and flocculation always needed as a water treatment stage? No.
In the majority of cases, these processes are used. They’re especially essential in wastewater treatment and are a necessary first step toward turning wastewater into potable water. However, they’re not always needed for drinking water treatment.
For instance, some drinking water supplies are groundwater (underground) sources. These water supplies are naturally filtered as water flows through layers of rocks and soils into the aquifer. There’s sometimes no benefit to using coagulation and flocculation to treat groundwater supplies.
To find out whether your drinking water or wastewater is treated with these methods, visit your local water treatment plant’s website or contact their customer support.

📑 Final Word
Most water treatment plants rely on coagulation/flocculation to significantly improve water quality and save time and money in the process of producing treated water. Coagulation and flocculation are an essential first step in wastewater treatment, removing the particles that reduce the effectiveness of water disinfection.
In most scenarios, the quickest easiest way to remove suspended particles is with these two water treatment methods. But it doesn’t end there.
Depending on the water source in question and the intended end result, further treatment stages will likely be needed to remove dissolved particles and kill microorganisms, making water safe for its intended use.
In a typical water treatment plant setup, coagulation and flocculation are followed by sedimentation, filtration, disinfection, storage, and finally, distribution.
❔ Coagulation And Flocculation: FAQ
What’s the difference between coagulation and flocculation?
The coagulation & flocculation processes achieve a very similar end result, but are unique in their own right. Coagulation is the chemical process of adding chemical coagulants to water and employing rapid mixing and slow mixing to disperse the chemicals throughout the water, neutralizing the charges of suspended particles and encouraging them to clump together. Flocculation is a physical process involving adding agents, called flocculants, that further encourage particles to bind together, promoting clumping of the particles that were neutralized by coagulation.
What does coagulant do?
The role of a coagulant is to neutralize the charge of suspended particles in water, which enables them to clump together and be more easily removed from the water, especially when a flocculant is added.
What is the purpose of a flocculant?
The purpose of a flocculant is to promote the clumping of suspended particles (floc formation) that have been neutralized by coagulation. it’s much easier to remove suspended solids when they’re clumped together compared to when they’re separate in water and repelling one another.
What is the difference between coagulant and coagulant aid?
A coagulant is a chemical that is used to neutralize fine particles and encourage them to clump together in the coagulation/flocculation process. A coagulant aid is a different material or chemical that is needed to modify or assist coagulation after the coagulant has been added. For example, a coagulant aid may be added to make flocks tougher to prevent them from breaking up during mixing and settling.
Is alum a coagulant or flocculant?
Alum is a coagulant, NOT a flocculant. It’s classed as a coagulant because it neutralizes suspended particles, which is the main purpose of coagulation. Alum is an inorganic coagulant, like sodium aluminate, ferrous and ferric sulfate, and ferric chloride.

Helpful materials. Thanks mum
Im trying to clarify outdoor shower water that contains alot of fine silt which is of an alkaline nature. I have a filter system which will electrically sanitize and filter down to 2 microns down stream of this process what is best coagulant/ floculant. also what would be best way to filter the precipitate?
Just for one point of use? An inline sediment filter (or step down series of) to filter the precipitate
This is a good information summarize the WT process, Thanks
Glad it was helpful!
Thank you for this report. Really helped me to finalize my Principles of Environmental Term Report!!
Glad it was helpful!
I learned something awesome today!
thank youuuuuuuuu!!🥳
Glad to hear it!! Thanks for reading and your comment