Alkalinity is one of the key water parameters that’s considered when evaluating water quality.
Here, we’ve answered the question, “What is alkalinity in water?” You’ll learn what alkalinity measures, why alkalinity is important, what affects alkalinity, and much more.
📌 Key Takeaways:
- Alkalinity is a measure of water’s capacity to neutralize acids.
- Alkalinity buffers help so keep pH stable, preventing fluctuations in a solution’s pH range, because of their acid neutralizing capacity.
- Water alkalinity is important because it helps to determine water’s likelihood to cause aesthetic issues, pose health risks, and threaten aquatic life and ecological systems.
Table of Contents
- 🚰 What Is Alkalinity?
- 🆚 Alkalinity Vs Acidity
- 🔎 What Affects Alkalinity?
- 🤔 Why Is Alkalinity Important?
- 📏 How Is Alkalinity Measured?
- 🗺️ Alkalinity Of US Surface Waters
- 🩺 Potential Health Risks Of Alkalinity
- 📉 Are There Drinking Water Standards For Alkalinity?
- 🧪 How To Test Your Water’s Alkalinity At Home
- 🔀 How Else To Determine Water’s Alkalinity
- ⚗️ How To Treat Water Alkalinity
- 📑 Final Word
- ❔ FAQ
🚰 What Is Alkalinity?
Alkalinity is a water property that determines a water source’s ability to resist pH changes that would result in acidity.
The most common alkalinity components are carbonate, hydroxide, and bicarbonate ions. These materials can be obtained through their mineral origin, as well as microbial decomposition of organic matter and the dissolution of carbon dioxide.
Carbonate alkalinity, hydroxides, and bicarbonate alkalinity all contribute to total alkalinity. Boric acid also contributes to total alkalinity, but not to carbonate alkalinity.
Most tap water supplies in the US are alkaline. Alkalinity is useful in drinking water because it protects pipes and plumbing, and has several health benefits compared to acidic water.

Alkalinity Vs pH
Alkalinity and pH are often confused, but while they’re related, they’re not the same thing.
Alkalinity is a measure of the amount of acid that can be added to water before its pH begins to change.
pH, on the other hand, is a measurement of the concentration of hydrogen ions in water, which determines its acidity.
Alkalinity Vs Hardness
Water hardness and alkalinity are two water parameters that also get confused, especially since water experts commonly report the alkalinity of water as mg of calcium carbonate equivalent, or CaCO3/L equivalent.
Hardness is a measure of how much calcium carbonate water contains. The presence of calcium plays a role in water’s alkalinity – calcium-rich water is more alkaline than water containing no calcium.
🆚 Alkalinity Vs Acidity
So, how does alkalinity compare to acidity in water?
Alkalinity is water’s acid-neutralizing capacity.
You might have heard alkalinity referred to as “the protector of the stream” because in surface water, it prevents the water from being affected by sudden pH changes caused by increased acid deposition in the atmosphere, and pollution from groundwater discharge, runoff, and industrial waste.
Some surface waters and groundwater sources have low alkalinity, which means they’re more susceptible to the effects of pollution from organic acids and acid rain. On the other hand, groundwater located in areas with a lot of limestone typically have a high alkalinity of 150 mg/L or more.
Acidity is the opposite of alkalinity – it’s water’s base neutralizing capacity.
Water’s corrosiveness and response to chemical reactions and biological processes are influenced by acidity.
🔎 What Affects Alkalinity?
There are several factors that affect the alkalinity of a water source.
Surface waters, like lakes and streams, are influenced by the presence of the rocks and soils that the water comes into contact with.
Precipitation from the area of water surrounding the river or lake (called a watershed) eventually ends up in the water due to runoff. As this runoff travels into the water source, it picks up minerals from rocks like limestone, which raise the water’s alkalinity and pH.
Not all regions have alkaline rocks. Regions with a lot of granite rocks, which are lower in alkalinity, typically also have water sources with lower alkalinity and pH.
Human activities also affect water’s alkalinity. For instance, surface water sources in a suburban area could have a high alkalinity due to the use of limestone on soils in the area to raise the soil’s pH and make it more suitable for growing certain plants.
🤔 Why Is Alkalinity Important?
There are a few reasons why alkalinity is important in our water sources.
Determines The Health Of Surface Waters
First, in natural water supplies, alkalinity is an important tool that’s used to determine the water’s health and welfare.
Organisms that live in lakes, rivers, and streams are adapted to specific water conditions. Many of these organisms are at risk of stress, disease, and death if these water conditions change, simply because they haven’t evolved to be resilient to changes in their environment.
The likes of acid rain and chemical spills can all be traced back to human activity, so our natural water sources originally remained consistent and didn’t change quickly over a short period – ideal for the organisms living in the water.
Now, however, it’s unfortunately common for our water bodies to be exposed to chemicals in wastewater and acid rain, which may rapidly change the water’s acid or base balance, lowering its pH. If this happens, it puts the aquatic organisms living in the water body in danger.
So, since we know that water with high alkalinity is less susceptible to changes caused by this type of pollution, being aware of a water source’s alkalinity can help us to determine its likelihood to experience sudden shifts in pH if exposed to acids and chemicals.

Determines Water’s Corrosiveness
The lower a water source’s alkalinity, the fewer alkaline minerals it’s likely to contain, the lower its likely pH, and the more likely it is to be corrosive.
Corrosive water causes a host of issues in a home plumbing system.
When water with a low alkalinity travels through pipes, it pulls some of the contaminants from the pipe materials into the water supply.
So, if you have low-alkalinity water, it’ll likely be contaminated with copper from your pipes. You’ll notice greenish-blue stains on your fixtures if you have an issue with copper pipe corrosion in your home.
Heavy metals from your pipes affect your water’s taste and have potential health effects, depending on the concentration of metals present.
So, alkalinity is important for drinking water supplies because it determines water’s likelihood to damage home plumbing systems and cause health problems.
Corresponds With Water Hardness
You might assume that high-alkalinity water is favorable, and it is – but it’s possible for water to be too alkaline, which poses its own set of unique problems.
We know that alkalinity is associated with water hardness. Generally, the more alkaline a water supply is, the more likely it is to contain calcium carbonate minerals – which, in turn, increases water hardness.
Depending on its exact properties, hard water may have a bitter taste and a chalky texture. It reacts with soap, forming a sticky substance called soap scum, and has been found to aggravate dry, itchy skin and hair conditions.
Studies have found that hard water also has a “tendency to corrode” as a result of hard deposits – mineral scale caused by the presence of calcium carbonate. Hard water with a higher alkalinity also forms limescale on fixtures and appliances, hindering their performance efficiency, reducing flow rate, and shortening their lifespans.
So, it’s helpful to know about water’s alkalinity when determining its likelihood to be hard, and therefore its likelihood to damage your plumbing as a result of mineral buildup.

📏 How Is Alkalinity Measured?
There are a few different methods of measuring alkalinity.
A common method is employed by the U.S. Geological Survey (USGS). This method involves taking a sample of water, then adding acid gradually to the water, checking its pH each time.
Scientists start by taking an initial pH reading of the water. Then, they add measured increments of an acidic solution, stirring the water and taking the pH each time. They continue to add increments of acid until the bicarbonates in the water are “used up” and no longer able to neutralize acid.
Once this happens, any further acid added to the water will reduce its pH, and the scientist can determine the water’s alkalinity by measuring how much acid was added to the water to result in the pH change.
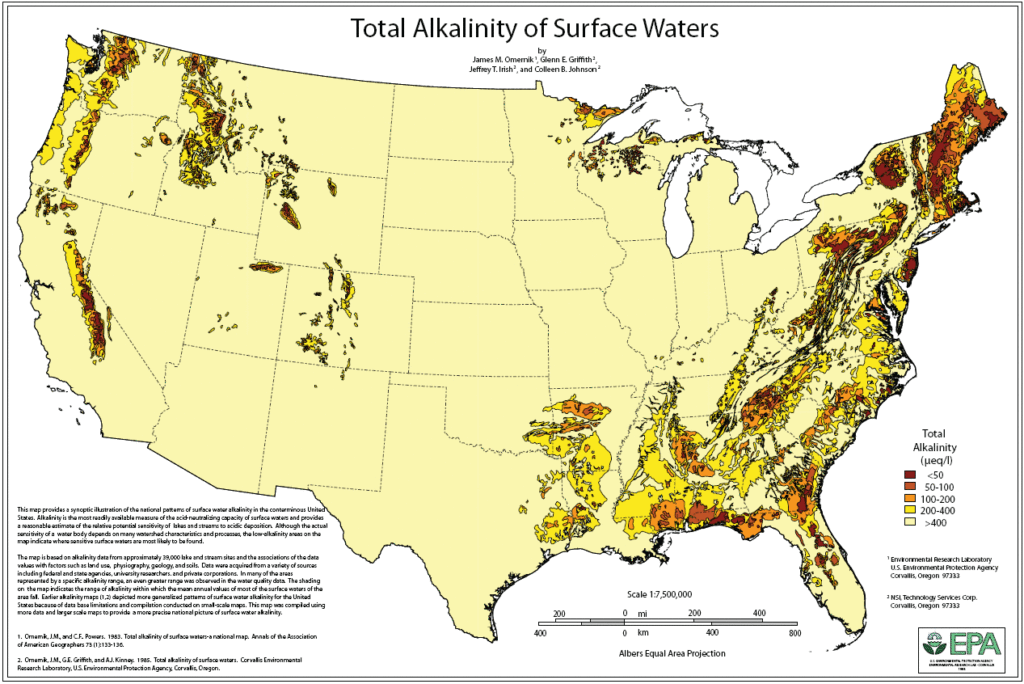
🗺️ Alkalinity Of US Surface Waters
Wondering whether your local surface waters are more acid or alkaline? Check out this EPA map of the total alkalinity of surface waters in the United States. The different-colored shading indicates the level of alkalinity in each location.
It’s a bit outdated, having been published in 1983, but it’s still a useful resource that gives us an idea of which parts of the US have very alkaline waters, and which have water with a low total alkalinity.
Keep in mind that the map doesn’t provide a thorough or complete overview of alkalinity in all the US states. It’s based on data from around 39,000 streams and lakes, which were considered alongside factors including soils, geology, and land use.
The authors of the map noted that water’s acid-neutralizing capacity and sensitivity depend on a number of characteristics of the watershed and processes, but the areas on the map with low-alkalinity shadings indicate the likely presence of sensitive surface waters.
🩺 Potential Health Risks Of Alkalinity
Alkalinity itself isn’t dangerous because it’s not a physical water contaminant or a pollutant with known health effects – it simply determines water’s susceptibility to pH changes as a result of chemical exposure.
Remember also that alkalinity is only one determiner of water quality. So, even if water’s alkalinity doesn’t indicate that it may pose certain health risks, other water quality parameters or the presence of certain contaminants may affect water’s safety to drink in other ways.
However, water’s alkalinity can be linked to the likelihood of its ability to cause certain health effects, and here’s how.
If water alkalinity is low, it’s more prone to corrosion of pipes, plumbing, and fixtures. The water is “hungry” and eager to take in minerals and metals, so it’ll leach what it can from its surroundings.
In the case of a drinking water supply, the water may pull metals from a plumbing system, increasing its metal content – not to mention damaging your pipes, causing aesthetic issues and leaks.
The health effects of drinking water containing these metals depends on exactly which metals are present.
For instance, if your home has copper pipes and high levels of copper are leached into your water, your tap water’s copper concentration might exceed the legal limit set by the Environmental Protection Agency, potentially putting you at risk of the health effects of excess copper ingestion.
Your risk of health effects is even greater if your home is old and contains lead pipes and plumbing. Lead is a highly toxic contaminant, and no amount of this metal is considered safe in water. So, if your water has low alkalinity and is leaching lead, you may experience symptoms of lead poisoning.
High alkalinity water has its own possible health effects, too. Alkalinity is connected to water hardness, so if you have very alkaline water, it’s likely also hard.
Hard water has several known skin and hair health effects, including increased risk of atopic dermatitis (a type of eczema that causes cracked, itchy, dry skin), breakouts, and dandruff.
However, despite its association with hardness, higher-alkalinity water is usually safer to drink than water with a low alkalinity.

📉 Are There Drinking Water Standards For Alkalinity?
There’s no legal limit for water alkalinity – high or low – but the Environmental Protection Agency has established a secondary drinking water standard for total dissolved solids (TDS).
While TDS and alkalinity are not the same, high levels of dissolved solids are associated with high alkalinity. So, in providing guidelines for TDS in water, the EPA inadvertently controls alkalinity.
The EPA secondary drinking water contaminant for TDS is < 500 mg/L.
Alkalinity is classified as low, moderate, and high when detected in the following ranges:
| Range | Alkalinity |
|---|---|
| < 20 mg CaCO₃/L | Low Alkalinity |
| 20 to 160 mg CaCO₃/L | Moderate Alkalinity |
| > 160 mg CaCO₃/L | High Alkalinity |
If your water has an alkalinity of less than mg CaCO₃/L, you will likely begin to notice aesthetic effects associated with the water’s corrosive properties.
🧪 How To Test Your Water’s Alkalinity At Home
The method of testing alkalinity requires precision and patience, and there aren’t many accurate at-home quick-results test kits that you can use to detect this water parameter.
There are water pH tests and pH meters, which you can use to get an idea of your water’s alkalinity – but we know that pH and alkalinity aren’t entirely the same.
The best way to get an accurate reading of the alkalinity of a water supply is with a laboratory test kit.
A laboratory test kit should include all the tools you need to take a sample of your water to ship to the lab for testing, including sample pot and the test instructions.
The average price of a laboratory test for alkalinity is $60-$120, depending on the testing process used and whether any additional parameters or contaminants are tested for at the same time.
To test your water’s alkalinity with a laboratory test, follow these steps:
- Order your test kit and wait for it to be delivered to your home.
- Follow the instructions in the test to take a sample of your tap water.
- Ship the sealed sample pot back to the laboratory.
- Wait to receive your test results (usually within 7-10 days) via email or post.

🔀 How Else To Determine Water’s Alkalinity
Don’t want to pay for a test to find out whether or not your water is alkaline?
There are a few other ways you can use to determine your water’s alkalinity without spending any money upfront.
The first is to look for signs of corrosion or hardness damage in your plumbing. If your water has a metallic taste and leaves blue or green stains on your fixtures, it probably has low hardness, pH, and alkalinity.
On the other end, if your water leaves white or gray deposits on surfaces, doesn’t lather well with soap, and leaves your skin feeling sticky after washing and showering, it probably has high hardness, pH, and alkalinity.
If your water alkalinity is too low, you may observe plumbing and fixture damage due to corrosion, including pinhole leaks and discoloration.
If your water alkalinity is too high, you may notice that your dishwasher and washing machine are less efficient, your water pipes are clogged or water flow is reduced, and limescale forms on your fixtures and appliances.
⚗️ How To Treat Water Alkalinity
As we mentioned earlier, alkalinity alone isn’t considered a problem, and it’s not a specific contaminant, so it can’t (and doesn’t need to) simply be removed.
However, you can install a water treatment system that will address a certain issue that relates to water alkalinity, such as limescale formed by excessive alkalinity, and an inability to remove a specific contaminant or control pH due to a lack of alkalinity.
The best type of water treatment for low alkalinity is an acid neutralizing system. This system raises the pH of the water, often by introducing minerals that boost its alkalinity.
Most water treatment processes, including anion exchange, iron/manganese removal, water softening, and reverse osmosis water filtration, reduce alkalinity as a byproduct, but this isn’t their main purpose.
For instance, a reverse osmosis system removes significant concentrations of TDS, and low-TDS water typically has a low alkalinity due to the lack of minerals that contribute to alkalinity.
This can actually cause problems in your home due to the corrosive properties of low TDS, low-alkaline water, which is why RO systems are typically sold as point-of-use systems, so the water travels through as little piping as possible before reaching your faucet.

📑 Final Word
Water alkalinity determines its susceptibility to leaching and corrosion in your home. Alkalinity varies from one location to the next, depending on geochemical and biological processes, evaporation-precipitation, human activities, and more.
If your water’s alkalinity is too low, you may need to resolve the issue by installing an acid neutralizer in your home.
👨🔧 Check out our ultimate guide to finding the best acid neutralizers for well water
❔ FAQ
What does alkalinity of water indicate?
Alkalinity of water indicates its buffering capacity and is determined by the presence of certain environmental factors – particularly geological factors – in the region in which the water source is located. Limestone, phosphates, and borates increase water’s alkalinity and buffering capacity.
Is high alkalinity in water good?
High alkalinity in water is usually preferable because it means that water is less susceptible to sudden pH and condition changes as a result of exposure to various chemicals in the environment. However, high alkalinity also corresponds to higher water hardness, which means water is more likely to form limescale deposits and have its own detrimental effects on plumbing and fixtures in our homes.
What is the difference between pH and alkalinity?
The difference between pH and alkalinity is that pH tells you whether a water source is acidic, neutral or basic, while alkalinity is a measure of the buffering capacity of the water. It’s possible to have high or low pH water that has minimal buffering capacity, so you can quickly alter its pH.
Is alkalinity safe in drinking water?
Yes, alkalinity is safe in drinking water. Alkalinity isn’t a chemical or a contaminant – it simply determines water’s susceptibility to pH changes as a result of chemical exposure. So, high-alkaline water is typically safer to drink than low-alkaline water because low-alkaline water has acidic properties that make it more likely to leach metals and other potentially harmful contaminants.
What is the normal alkalinity of water?
The typical range of alkalinity for drinking water is 20-200 mg/L, depending on its dissolved mineral content. Water on the higher end of this range is likely to be associated with hard water effects and limescale, while water on the lower end of this range is more likely to corrode pipes and leach metals and minerals from its surroundings.
