Water hardness is a measure of how much calcium and magnesium your water contains. To find out the total permanent hardness of your water, you need to combine the total calcium hardness and magnesium hardness.
Table of Contents
- 📲 Calculate Water Hardness
- 🚱 What is Water Hardness?
- 🆚 Temporary Vs Permanent Hardness
- 📟 How a Water Hardness Calculator Works
- 📐 Units of Measurement for Total Hardness in Water
- 📰 Water Hardness Categories
- 📖 How to Test for Water Hardness at Home
- 🔎 Analyzing Your Results
- 🧪 Treating Your Hard Water
- 🧠 Water Hardness Calculator: FAQs
📲 Calculate Water Hardness
You can get an estimate of your water hardness by using the water hardness calculator below.
Hardness(mg/L or ppm)
–
Hardness(grains per gallon)
–
Indication
–
🚱 What is Water Hardness?
Water hardness is defined by how many hard-causing minerals (namely calcium carbonate and magnesium) your water contains. Soft water contains low levels of minerals, while hard water contains high levels of minerals.
There are noticeable effects of using hard water in your home, including limescale buildup in pipes and appliances, a filmy residue on your skin, white spots on your glassware, and reduced appliance efficiency.
Hard water does have some benefits: calcium and magnesium minerals are important for human health. However, these minerals are found in plenty of foods, so they’re not essential in drinking water.

🆚 Temporary Vs Permanent Hardness
Temporary hardness and permanent hardness aren’t the same. We’ve outlined the key differences between the two below.
Temporary Hardness
Temporary water hardness is caused by dissolved calcium hydrogencarbonate (Ca (HCO3)2) and magnesium hydrogencarbonate (Mg (HCO3)2) in water. Both of these minerals dissolve when water is boiled. Temporary hardness can also be removed by adding lime (calcium hydroxide) in a process known as lime softening.
Permanent Hardness
Permanent hardness contains chlorides and sulfates of magnesium and calcium. Water is classed as permanently hard if it contains calcium chloride and/or sulfate, and/or magnesium chloride and/or sulfate. Permanent hardness is the measure of permanent calcium hardness and permanent magnesium hardness. These minerals don’t dissipate when water is boiled, so special treatment is required to tackle permanent hardness, such as an ion exchange water softener.
The below table highlights the key differences between temporary and permanent water hardness:
| Temporary Hardness | Permanent Hardness | |
|---|---|---|
| Caused By | Calcium hydrogencarbonate & magnesium hydrogencarbonate | Chlorides and sulfates of magnesium and calcium |
| Methods of removal | Boiling water, adding lime | Ion exchange water softener |
📟 How a Water Hardness Calculator Works
We now know that water hardness is present in two forms: temporary and permanent. Hard water calculators tell you the overall permanent water hardness using the following formula:
TOTAL PERMANENT HARDNESS = CALCIUM HARDNESS + MAGNESIUM HARDNESS
Based on the molar mass of calcium and magnesium, hardness is calculated with this equation:
Hardness = 2.497 (Ca) + 4.118 (Mg)
You can see from this equation that hardness is most strongly affected by fluctuations in magnesium than calcium.
📐 Units of Measurement for Total Hardness in Water
Water hardness is measured in several different units:
- Grains per gallon (GPG)
- Parts per million (PPM)
- Milligrams (of calcium) per liter (mg/L)
- Dgh or dH (usually measures the hardness of water in fish tanks)
Most commonly, GPG is used to measure water hardness. Occasionally, PPM and mg/L are also used to measure total hardness. These units are interchangeable, so 20 PPM of dissolved minerals is equal to 20 mg/L.
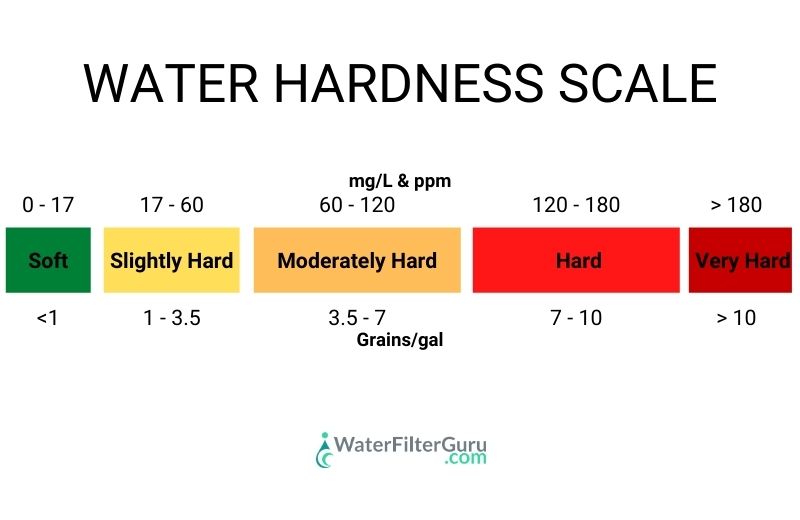
📰 Water Hardness Categories
You can work out how hard or soft your water is by comparing the total hardness measurement to the graph below:
📖 How to Test for Water Hardness at Home
There are a number of ways to test for water hardness at home, including using an at-home test kit, testing your water with a laboratory, or using the soap test.
Using an At-Home Test Kit
An at-home test kit is a quick, affordable means of testing for water hardness with a simple test strip concept.
Most at-home hardness test kits include:
- Test strips
- A color chart
- A sample pot (optional)
Most at-home test kits will give you a range of water hardness, in GPG or PPM, depending on the color of the test strip once you’ve dipped it in your water sample.

Testing With a Laboratory
A laboratory test is the most thorough test for hardness you can do for either a treated water supply or an untreated water supply.
Most laboratory tests come with:
- Labeled sample pots
- Packaging for posting
Once you’ve collected your samples and sent them back to the laboratory, the water will be professionally tested to determine its magnesium and calcium carbonate hardness. You’ll know your water’s exact hardness, in PPM, mg/L, or GPG, from this type of test.

Using the Soap Test
The soap test is the least accurate way to measure your water hardness. It won’t tell you your exact water hardness levels – but it will tell you whether or not you have hard water. Soap reacts with soft water much better than it does with hard water, so a soap test can teach you about your water chemistry.
For this test, you’ll need:
- A clear water bottle
- Water from your faucet
- Dish soap
Fill the bottle to about three-quarters full, then add a drop of dish soap. Shake the bottle, then take a look at the water. If the water is milky white, with no distinct layer of bubbles on top, it’s hard. If the water is clear, with a layer of bubbles on top, it’s soft.
🔎 Analyzing Your Results
Once you’ve received your water hardness results, you can analyze your findings and decide whether you need a water treatment system for your home.
Water with a measurement of 0 to 5 GPG, or less than 60 PPM or mg/L of hardness is considered soft to moderately hard. If your water has a reading of less than 5 GPG or 60 PPM or mg/L, you’re unlikely to need a water softener.
Water with a measurement of 5 to 10 GPG, or 180 PPM or mg/L of hardness is classed as moderately hard to very hard. These levels of hardness are likely to cause damage in your home, and would benefit from water softening treatment.

🧪 Treating Your Hard Water
The best method of treatment for hard water is a water softener.
Water softeners physically remove calcium and magnesium ions and replace them with sodium ions in a process called ion exchange. They’re installed at the point where your main water pipe enters your home, providing protection against dissolved minerals throughout your entire home.
Water conditioners are the second-most effective means of minimizing the side effects of hard water. However, water conditioners don’t physically remove minerals or reduce total hardness – they just crystallize the existing hardness minerals, preventing them from causing scale.
🧠 Water Hardness Calculator: FAQs
How do I calculate water hardness?
The easiest way to calculate water hardness is to use a water hardness calculator tool. Simply enter the calcium carbonate hardness in mg/L, and then enter the magnesium hardness in mg/L. Click “calculate”, and the calculator will return the total hardness in mg/L of CaCo3.
Is 220 PPM hard water?
Yes. Anything over 180 PPM is considered very hard water, so if you measure hardness as 220 PPM, it’s a sign that your water is hard.
What is normal hardness in water?
There is no normal hardness in water. Your water hardness is determined by the geology of where you live and how your public water is treated.
How do you convert TDS to hardness?
There is no completely accurate way to convert TDS to hardness. However, if you just want an estimate, you can divide the PPM measurement of the TDS by 10. The number you’re left with is the rough hardness value of your water.
What are the units of water hardness?
The most common units of water hardness are GPG (grains per gallon), mg/L (milligrams of calcium per liter, and PPM (parts per million). mg/L and PPM are interchangeable.
How much hardness does soft water contain?
Soft water contains less than 1 GPG or 0 – 17 PPM or mg/L of total magnesium and calcium carbonate hardness. If your water contains this amount of hardness, you’re unlikely to need a water softening system.
Is hard water safe?
Yes, magnesium and calcium carbonate hardness are safe for human consumption. Most people want to calculate water hardness because they’re considering buying a water softening system to manage the aesthetic issues related to high magnesium and calcium content, such as damage to your water heater, plumbing, and appliances.

